How do I learn what chemical element reacts with what other element?
I already know: structure of the atom, aufbau principle, electronegativity, what else do I need?
————————————————————————————————————
You’re already naming the right foundations. To reliably predict what reacts with what, you build a layered mental model. Here’s the complete framework, ordered from most fundamental → most practical.
1️⃣ Atomic structure & quantum rules (the why)
a) Electron configuration (Aufbau + Pauli + Hund)
- Aufbau → filling order
- Pauli → max 2 e⁻ per orbital, opposite spin
- Hund → maximize unpaired electrons
This tells you:
- Valence electrons
- Stability (filled vs half-filled shells)
- Likely oxidation states
Elements react to complete, empty, or stabilize their valence shell.
b) Valence electrons (MOST important early shortcut)
- Group number ≈ valence electrons (main-group elements)
- Predicts bonding patterns immediately
| Valence e⁻ | Tendency |
|---|---|
| 1 | Lose 1 (alkali metals) |
| 2 | Lose 2 |
| 6 | Gain 2 |
| 7 | Gain 1 |
| 8 | Inert |
2️⃣ Electronegativity (WHO pulls electrons)


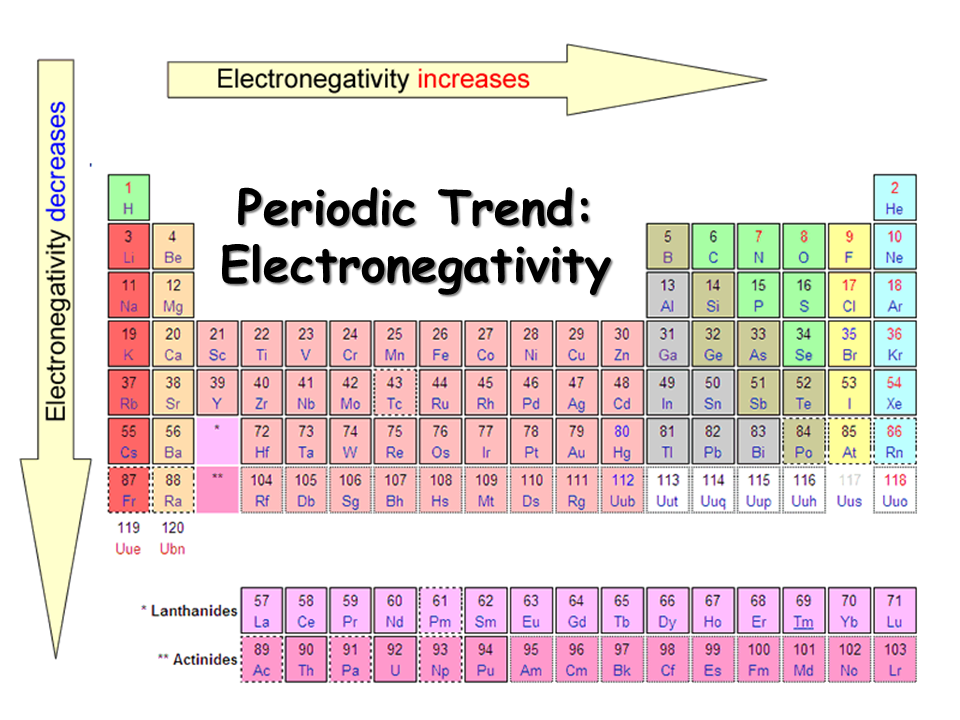
Electronegativity answers:
- Who takes electrons
- Bond polarity
- Ionic vs covalent character
Rules of thumb:
- ΔEN > ~1.7 → mostly ionic
- Small ΔEN → covalent
- F, O, N, Cl are aggressive electron pullers
3️⃣ Ionization energy (WHO lets go easily)
- Low IE → element loses electrons easily
- High IE → holds electrons tightly
Explains:
- Why Na reacts violently, Ne doesn’t
- Why metals oxidize
Trend:
- ↓ down a group
- ↑ across a period
4️⃣ Electron affinity (WHO wants electrons)
- How much energy is released when gaining e⁻
- High for halogens
- Complements electronegativity
5️⃣ Orbital overlap & geometry (HOW they bond)

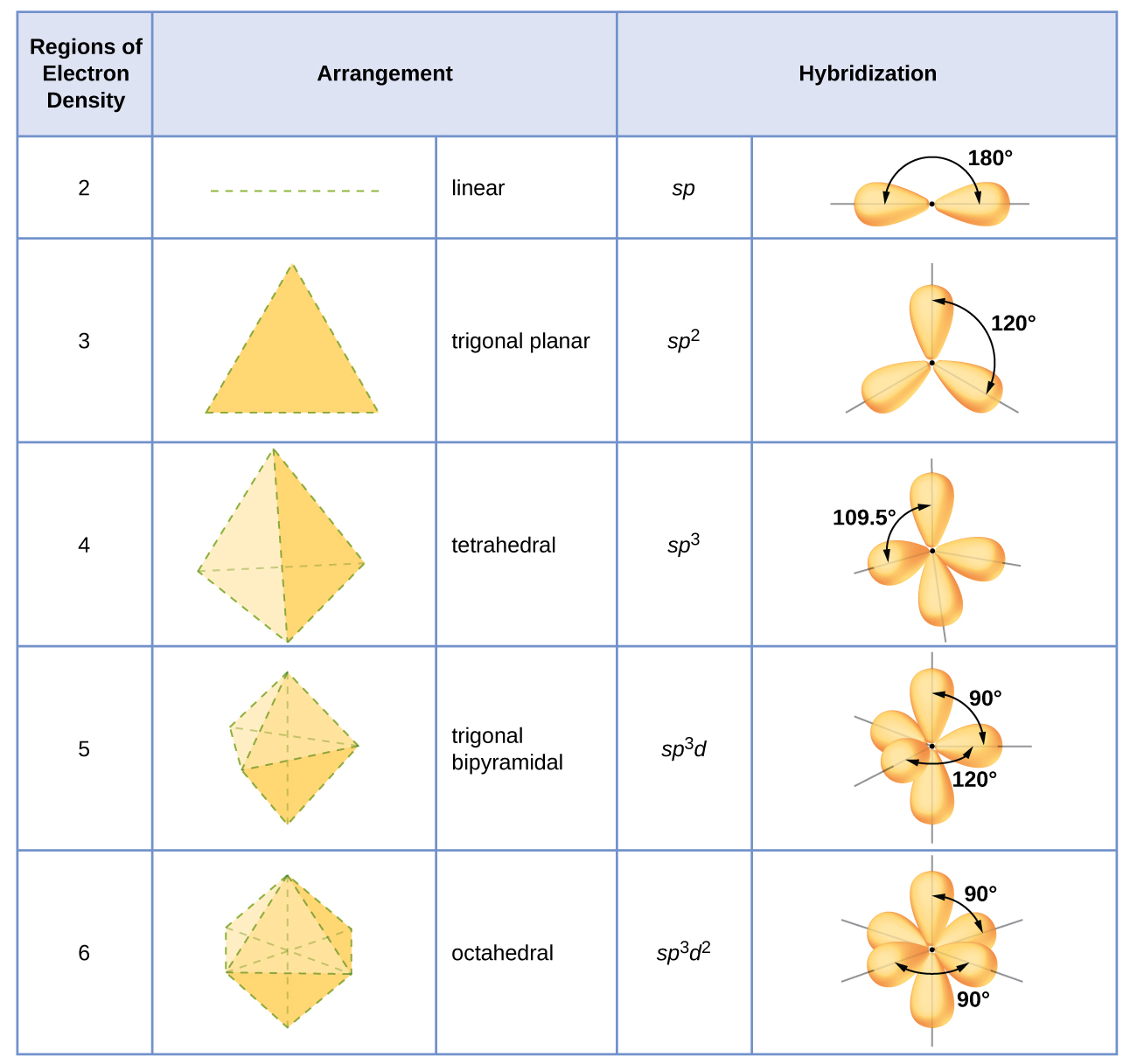

- s–s, s–p, p–p overlap quality
- Hybridization: sp, sp², sp³
- Determines bond strength & shape
6️⃣ Periodic trends (PATTERN recognition)
You should internalize:
- Atomic radius
- Metallic character
- Reactivity trends
Example:
- Alkali metals → more reactive down group
- Halogens → more reactive up group
7️⃣ Oxidation states & redox logic
Every reaction is secretly:
electrons moving
Ask:
- Who is oxidized (loses e⁻)?
- Who is reduced (gains e⁻)?
This alone predicts:
- Metal + non-metal reactions
- Corrosion
- Batteries
- Combustion
8️⃣ Thermodynamics (WILL it happen?)
Even if something can react, ask:
- ΔG < 0 ? (spontaneous)
- Enthalpy vs entropy
This explains:
- Why some reactions need heat
- Why others explode
9️⃣ Kinetics (HOW FAST?)
- Activation energy
- Catalysts
- Temperature
- Surface area
Explains:
- Why diamond doesn’t burn easily
- Why radicals (•OH) are insane
10️⃣ Special cases you eventually memorize
- Noble gases (full shells)
- Transition metals (d-orbitals complicate)
- Radicals
- Acids & bases
- HSAB theory (hard–soft interactions)
🧠 The mental decision tree
When you see two elements:
- Valence electrons?
- Big electronegativity difference?
- Metal vs non-metal?
- Who loses e⁻, who gains?
- Orbital compatibility?
- ΔG negative?
- Kinetics reasonable?
If yes to most → reaction likely.
📘 How to actually learn this (practical plan)
Phase 1 (fast intuition)
- Memorize:
- Periodic table layout
- Valence electrons
- EN trends
- Practice predicting bonds before checking answers
Phase 2 (deep understanding)
- MO diagrams
- Hybridization
- Redox balancing
- Thermodynamics basics
Phase 3 (expert intuition)
- Reaction mechanisms
- Transition metals
- Radical chemistry
- Surface chemistry
One-sentence truth
Chemistry is electrons trying to reach lower energy states — everything else is bookkeeping.
If you want, next I can:
- Build a step-by-step learning roadmap
- Give you “reaction prediction drills”
- Connect this directly to hydroxyl radical chemistry (which you’ve been circling lately)
Just tell me how deep you want to go.
